Chris Richardson
President & CEO
Keystone Heart
Protecting the brain during
Cardiovascular Procedures
Transcatheter aortic valve replacement (TAVR) continues to be an effective and less invasive treatment option for patients suffering from severe aortic stenosis (AS) compared to open surgery. Although an excellent option in treating aortic stenosis, TAVR is not without its complications; cerebrovascular accidents are one of the most important and clinically significant adverse events for patients undergoing this procedure.
Israel and Florida-based Keystone Heart has made it their company mission to develop and manufacture novel devices that protect the brain from emboli to reduce the risk of brain infarcts during transcatheter aortic valve replacement (TAVR) along with other structural heart procedures. "During these cardiovascular procedures, debris from the aortic valve, ascending aorta and other sources may embolize and cause cerebral infarction. Embolic brain lesions resulting from these procedures may lead to potentially devastating outcomesùstroke, dementia and cognitive decline. Moderate to mild brain injuries, which are caused by new lesions in the brain, can affect the patient's processing speed, executive function, and fundamental skills such as memory, language, and balance. These lesions may be related to changes in the way the brain functions or processes information, and lesions in the brain stem can impact basic body functions such as breathing, swallowing, heart rate, blood pressure, consciousness, and whether one is awake or fatigued. The location of these lesions determines the damage and clinical symptoms, and where a lesion may occur is unpredictable," states Chris Richardson, president and CEO of Keystone Heart.
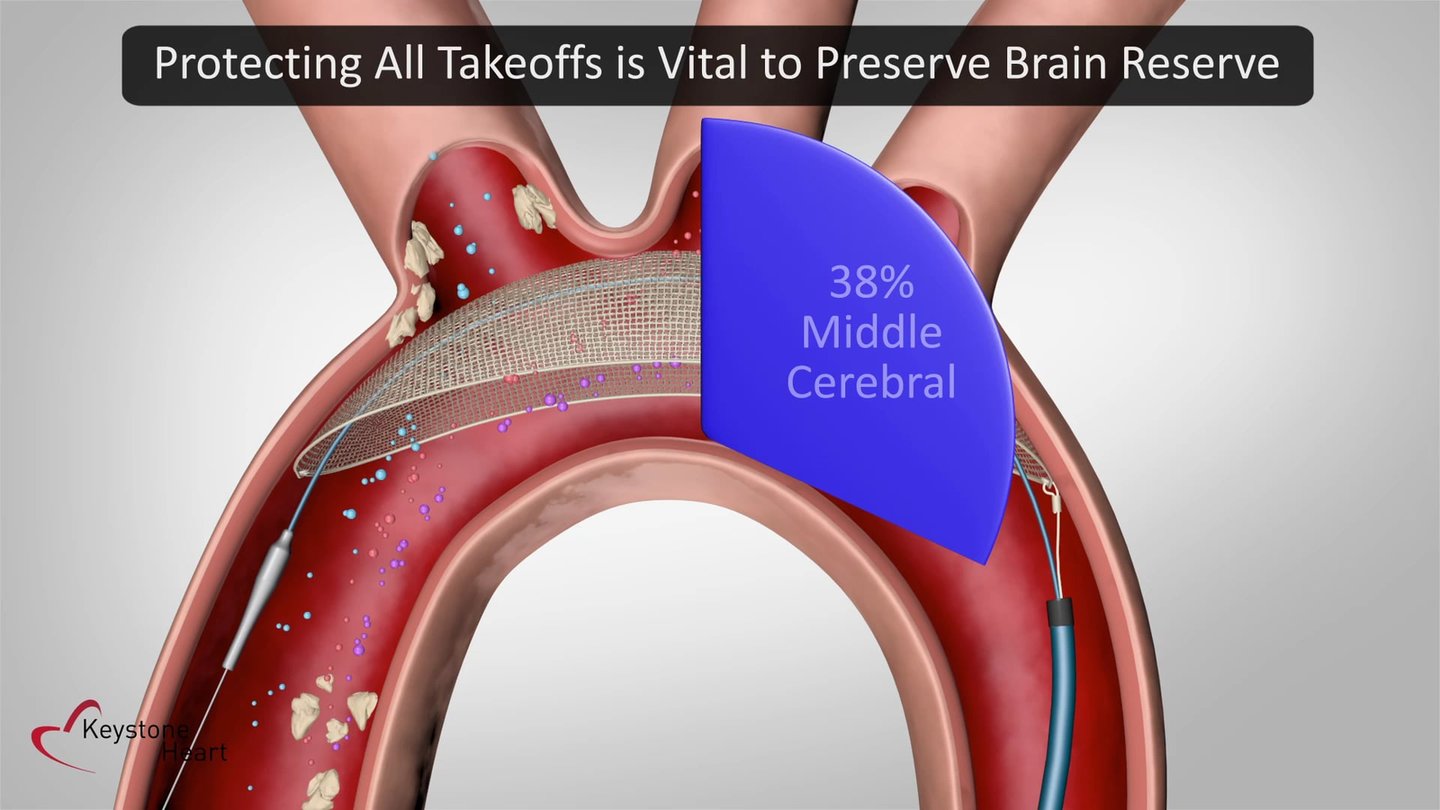
The team at Keystone Heart has designed TriGUARD 3, the first Cerebral Embolic Protection Device designed to provide complete coverage to all brain regions for patients undergoing cardiac procedures.
Over the next several years, Keystone Heart has invested in additional studies and device modifications to strive toward demonstrating the clinical benefits of cerebral embolic protection. Today, a multi-center, randomized clinical trial evaluating Keystone's latest device, the TriGUARD 3 CEP device, is currently underway. The company anticipates receiving FDA approval for teh U.S market and CE mark approval for Europe by the end of 2019.
"TriGUARD 3 underscores Keystone Heart's dedication to excellence, determination to provide a total solution for patients undergoing TAVR procedures and focus on patient safety."
FACTS: TAVR
1. Number of TAVR procedures conducted annually worldwide has increased exponentially over the past 6 years.
2. A total of 13 studies conducted in the US and Europe have shown that a median of 80% of patients have new brain lesions following TAVR.
3. In recent research from leading US institutions, the NeuroTAVR study demonstrated that 94% of patients had new lesions in the brain following the TAVR procedure, one out of four have some neurologic impairment after TAVR, and four out of 10 have some neurocognitive worsening one month after TAVR when compared to pre-TAVR scores.
4. There will be approximately 125,000 TAVR procedures conducted this year worldwide.
According to Richardson, “Upon receiving regulatory approval, the TriGUARD 3 CEP device will be the only commercially available Cerebral Embolic Protection Device delivered transfemorally and designed to protect all cerebral aortic arch vessels with no anatomical restrictions or manipulation of the cerebral vessels. The TriGUARD 3 CEP device is designed to help interventional cardiologists and electrophysiologists protect the brain during cardiovascular procedures. The innovative Nitinol frame with dome shaped mesh deflector is designed to 'self-seat' and conform to all aortic arch anatomies while providing stability to the deflection filter. The TriGUARD 3 CEP device deflection filter allows adequate blood flow to the brain while diverting emboli downstream.”
In December 2018, Venus Medtech (Hangzhou) Inc., the preeminent Chinese transcatheter heart valve company, announced it has closed its merger with Keystone Heart. "Our partnership is allowing us the unique opportunity to improve the lives of patients undergoing structural heart procedures with cerebral embolic protection today while working closely with Venus Medtech to bring new and cutting-edge therapies into the US & European markets tomorrow" remarks Richardson, in his closing comments.