Biotronik
German medtech for infrequent arrhythmias
By Gergana Koleva
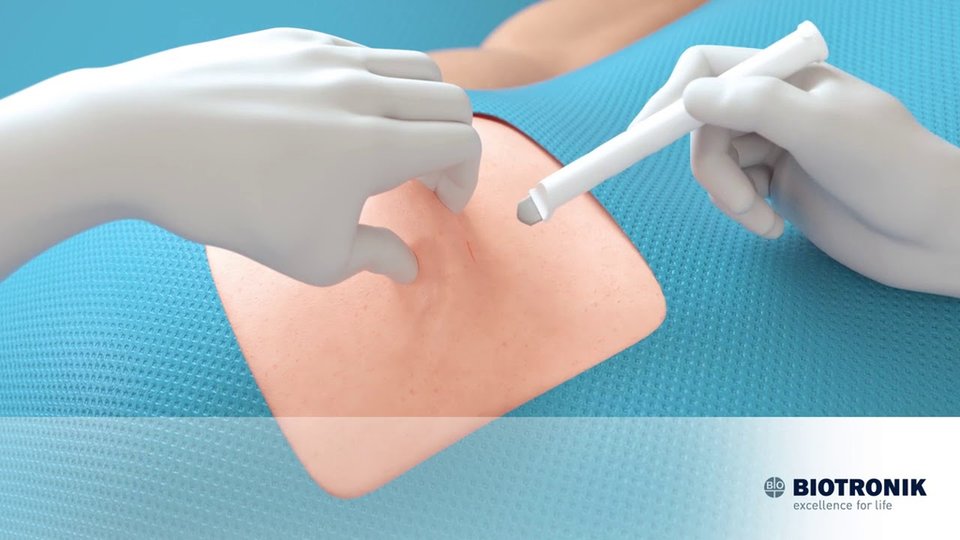
Berlin-based BIOTRONIK is setting a high bar for players in the implantable cardiac monitoring innovation game. Combining device miniaturization with unrivalled signal fidelity and intelligent memory management, its BIOMONITOR III and IIIm are two of the market’s most advanced tools for the detection of infrequent arrhythmias.
A privately owned, independent company with offices in more than 50 countries and R&D and manufacturing sites in Germany, Switzerland and the U.S., BIOTRONIK covers a lot of ground geographically and technologically. It has been churning out devices at the vanguard of cardiovascular and endovascular medical technology since its founding in 1963 and in the past few years has had its sights laser-focused on perfecting one particular invention: the BIOMONITOR ICM. This injectable monitor offers minimally disruptive beneath-the-skin placement and industry-leading longevity, but what really sets it apart from other remote cardiac monitoring devices are its highly advanced capabilities for detecting infrequent arrhythmias such as atrial fibrillation (AFib), bradycardia, sudden rate drop, high ventricular rate (HVR), and asystole.
“We know external monitors, wearables, and even the popular `Holter´ monitors all have one thing in common – they cannot bypass the most significant factor of all: the human factor,” says Ryan Walters, president of BIOTRONIK, Inc. Walters is referring to routine situations in the life of remotely monitored cardiac patients, such as manipulating handheld cardiac monitors or even showering, that render long-term uninterrupted monitoring impractical. “When patients are faced with asymptomatic arrhythmias or infrequent episodes, external monitoring can become impractical or burdensome to lifestyle.”
A trendsetter in the ICM space
The BIOMONITOR III and IIIm devices embody two trends revolutionizing the remote cardiac monitoring (RCM) space. One of them is a strong preference among clinicians for extended-duration cardiac rhythm monitoring devices due to their superiority in detecting infrequent disturbances. By definition, such arrhythmias do not necessarily manifest within the relatively short monitoring periods that external RCM devices such as patches, harnesses, and event monitors cover. Detecting them is where ICMs excel, as they typically provide several years´ worth of cardiac rhythm data – and the BIOMONITOR IIIm, with its 5.5 years´ worth of continuous data tracking, is at the top of that list. Its longevity makes it especially well-suited for patients with recurring unexplained syncopes, AFib, or cryptogenic stroke because it provides physicians with longitudinal data that facilitate detection of such arrhythmias and assist with diagnosis.
Another trend the BIOMONITOR encapsulates is its compatibility with MRI scans. BIOTRONIK’s first-generation BIOMONITOR device, released in 2013, already included ProMRI technology, which allows patients with heart rhythm disorders to safely undergo MRI scans. The latest iteration builds on that feature by accelerating access to MRI scanning through its 1.5T and 3.0T full-body MR conditional with no post-injection waiting period limitations – another aspect of the cardiac patient journey that external RCM devices are generally unable to resolve.
“We need patient compliance for monitoring to be done successfully. But due to the constraints of everyday life, external monitors can typically only deliver low diagnostic yields, leading patients on longer and more complicated journeys, which can increase the overall cost of care. BIOMONITOR III and IIIm are changing that,” says Walters.
German design at its finest
Evidence-backed excellence
The technical and design superiority of BIOTRONIK´s third-generation BIOMONITOR platform shines through in its unique, 70mm long sensing vector, which enables larger signal amplitudes with significantly less noise than the industry average. Featuring a flexible antenna and fractal coating for optimal sensing, the BIOMONITOR is designed to optimize detection accuracy. Unlike other ICMs on the market, BIOTRONIK´s device does not use secondary or tertiary signal processing filters, which are unnecessary due to the higher signal fidelity achieved via the long vector design and the resultant accurate and timely arrhythmia detections.
As it collects and stores arrhythmia data over time, the BIOMONITOR´s Intelligent Memory Management system ensures that less frequent and possibly more severe heart disturbances, such as asystole or ventricular tachycardia, are not overwritten in favor of more common ones such as AFib – a design flaw common to some extended-use cardiac monitoring devices. In fact, the device is programmed so that up to three episodes of every type of arrhythmia are automatically overwrite-protected, beyond any additional episodes stored over time. Its algorithms are also capable of recording and storing different types of arrhythmia that occur simultaneously, in addition to having memory allocated solely to patient-triggered episodes.
The BIOMONITOR system is completed by an optional handheld device for patients, which they can use to trigger symptomatic episode recordings, and with a smartphone-sized CardioMessenger Smart mobile unit that transmits the ICM data to BIOTRONIK´s Home Monitoring platform, accessible by the patient´s physician.
“We need patient compliance for monitoring to be done successfully. But due to the constraints of everyday life, external monitors can deliver a low diagnostic yield, leading patients on longer and more complicated journeys, which can increase the overall cost of care. BIOMONITOR III and IIIm are changing that.” – Ryan Walters, President, Biotronik
With a growing number of ICM patients being managed remotely, clinicians and healthcare providers are well aware that any ICM device is only as good as its remote monitoring system. Two of the most common issues that arise with such technologies are sensitivity and false positive rates, which effectively create the basis for accurate diagnosis, patient care, clinical workloads, the need for any additional external monitoring services, and potential further cardiac interventions.
The BIOMONITOR´s superiority in addressing these weaknesses has been tested in a number of clinical studies. The most recent of those, the BIO|CONCEPT.BIOMONITOR III study, was conducted in 2019 among 45 patients at high risk of developing clinically significant cardiac arrhythmias, with the objective of assessing the device´s sensing quality measured as the extent of the R-wave amplitudes and P-wave visibility it is capable of achieving. It delivered an impressive result: 0.70mV in the case of the former and 89% in the latter, observed across all cardiac cycles. In addition, according to proprietary company data, the BIOMONITOR´s positive predictive value for detecting AFib episodes is an impressive 98.5%, while preserving AFib episode sensitivity at 99.1%. By comparison, a different study of 559 patients implanted with the market-leading ICM device found false positive rates ranging from 46% to 86%, depending on the type of arrhythmia.
As far as the BIOMONITOR´s data transmission fidelity, the BIO|CONCEPT.BIOMONITOR III study demonstrated a 98% success rate in daily data transmissions to BIOTRONIK´s Home Monitoring platform. While impressive on its own, this rate is even more compelling when compared with the market leader´s 12% data transmissibility of all detected events within the first 24 hours following event detection. Although BIOTRONIK is a company with a rich and consistent history in medical device development, the image of the nimble start-up overtaking established players may not be out of place here.