Medtronic
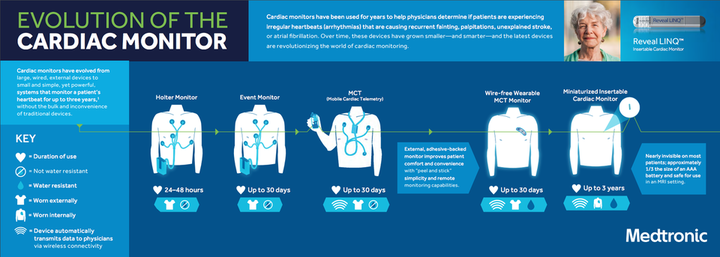
On a scale from Holter to implantable wireless cardiac monitor – bypassing event monitoring, mobile cardiac telemetry, and wire-free wearable MCT – Medtronic is pushing off the charts with their newest insertable cardiac monitor on the market.
Driving Insertable Cardiac Monitor (ICM) Innovation Since the Nineties
By Gergana Koleva

“The availability of remote monitoring data has enabled significant benefit for patients and providers. However, there is still a burden on the care team to respond to these data to enable efficient review and timely actions. Through the Reveal LINQ ICM with TruRhythm Detection, we saw an opportunity to improve the efficiency of data management and enhance the patient and provider experience.”
– Amaza Reitmeier, Vice President of Marketing, Cardiac Rhythm and Heart Failure Diagnostics, Medtronic
The Medtronic LINQ II insertable cardiac monitor (ICM) is the veteran devicemaker´s latest iteration of its pioneering line of cardiac monitoring devices. The technology arrived on the scene in 1998 with the world´s first Reveal ICM, whose success the company revisited a decade later with the introduction of the first ICM with a built-in atrial fibrillation (AF) algorithm. In 2014 it released the Reveal LINQ ICM, the world´s smallest ICM. And since 2017 it has taken its vision of cardiac monitoring to new heights by upgrading the LINQ ICM with intelligent new capabilities and self-learning AF algorithms that effectively convert it into an implantable cardioverter defibrillator (ICD) by enabling physicians to act instantaneously in case of an emergency.
“The availability of remote monitoring data has enabled significant benefit for patients and providers. However, there is still a burden on the care team to respond to these data to enable efficient review and timely actions. Through the Reveal LINQ ICM with TruRhythm Detection, we saw an opportunity to improve the efficiency of data management and enhance the patient and provider experience,” says Amaza Reitmeier, vice president of commercial marketing for Cardiovascular Diagnostics & Services at Medtronic.
“Over the past 20 years Medtronic ICMs have monitored more than one million patients across cryptogenic stroke, syncope, and AF management, and have shown a superior diagnostic yield leading to informed medical decisions.”
How it works
Clinically meaningful
Cleared by the U.S. Food and Drug Administration in February 2017, the Reveal LINQ with TruRhythm Detection is implanted just beneath the skin through a small incision in the upper left side of the chest, where it subcutaneously records and stores ECG data to detect abnormal heartbeats. It comes equipped with an extra sensing filter that analyzes rhythms for possible premature ventricular complexes (PVCs) or small-amplitude R-waves in bradycardia and sinus pause, as well as with a self-learning AF detection algorithm that calibrates its own threshold as it “observes” patients´ heartbeat patterns over time, making it especially relevant for people with sinus arrhythmia. The cumulative result of these enhancements has been a drastic reduction of false positives for bradycardia by 95%, for pause by 47%, and for AF episodes by 49%, compared to the original Reveal LINQ ICM, Reveal LINQ ICM with TruRhythm Detection’s predecessor.
“The AF algorithm analyzes the electrical signal between two R-waves to determine if a P-wave is present. In cases of suspected AF and in the existence of a P-wave, the Reveal LINQ with TruRhythm Detection rejects the rhythm as AF,” explains Reitmeier. “AF rejection due to P-waves is used as a self-learning mechanism, making the misclassification of AF in the same patient harder in future suspected AF instances with a minimal loss of true AF episodes.”
The device, which measures about one-third the size of a AAA battery and is safe for use in MRI settings, is indicated for patients with clinical indications or at increased risk of cardiac arrhythmias, or for those who experience transient symptoms such as palpitations, syncope, and chest pain that may suggest arrhythmias. It provides continuous wireless data monitoring and storage for up to three years – an engineering feature that puts it head and shoulders above both traditional 30-day event monitors and more advanced but similarly time-bound wearable MCT monitors. The data are transmitted to a patient bedside monitor and onto Medtronic´s CareLink network, which allows physicians to view each patient´s cardiac activity. This enables them to monitor and diagnose patients remotely, as well as to react rapidly in case of an abnormal cardiac event.
The growing volume of data produced by such continuous long-term monitoring bring to the fore the unique value proposition of the Reveal LINQ ICM with TruRhythm Detection: its smart filtering and algorithm systems, which not only detect irregular heart rhythm with superior precision, but also reduce the amount of data healthcare professionals eventually have to sift through. According to Medtronic´s estimates, the improved data management results in 56% less clinician time spent reviewing routine data, compared with the Reveal LINQ ICM without TruRhythm Detection.
In July 2020, the FDA cleared the LINQ II™ ICM with remote programming options and improved longevity (4.5 years) compared to other ICMs. The device delivers enhanced accuracy to correctly detect abnormal heart rhythms, simplifying the diagnosis and monitoring of patients.
LINQ II gives physicians clinically relevant data to help diagnose underlying heart conditions and define treatment protocols for patients with atrial fibrillation (AF) or other abnormal heart rhythms. Additionally, clinicians spend 33 percent less time reviewing ICM transmissions, resulting in potential office efficiencies and reduced costs due to more streamlined workflows.
The LINQ II system offers patients a consistent way to experience ongoing connectivity between their device and physician, while potentially reducing the need for in-office visits – a benefit for both patients and physicians, especially during the current COVID-19 pandemic.
The merits of Medtronic´s ICM devices have been validated by a number of clinical studies that show their effectiveness in detecting previously unidentified AF.
Among the most eloquent findings are those of the REVEAL AF study, a prospective global five-year clinical trial whose results underscore the benefits of the Medtronic ICM devices´ capacity for long-term uninterrupted monitoring. Concluded and published in JAMA Cardiology in 2017, the study enrolled 446 patients who had tested negative for AF but who were considered to be at high risk for stroke or high risk for AF. Of those, 385 were implanted with a Reveal ICM device and all were followed for an average of 22.5 months to observe the potential onset of AF. Among this cohort, the study showed that the median time to AF detection was 123 days – more than four times longer than the period encompassed by standard 30-day monitors.
“Data show that ICMs provide superior diagnostic yield over conventional care. For example, physicians are 4.3 times more likely to reach a diagnosis with ICM in 12 months versus a one-time, 30-day monitor.Specific to atrial fibrillation, studies have shown that the longer a physician looks for AF, the more often it is found. Eighty-eight percent of cryptogenic stroke patients who had AF would have been missed if only monitored for 30 days, while 84.5 percent of high-risk patients with AF would have been missed if only monitored for 30 days,” says Reitmeier, enumerating findings from REVEAL AF and from some of the over 700 other published articles and abstracts that support the clinical rigor of Medtronic´s ICM devices. Overall, she says the studies demonstrate that the Reveal LINQ ICM technology has the highest rate of accuracy in arrhythmia detection among heart monitors, an impressive 99.4%.
What does all that mean for the future of cardiovascular care and disease prevention, and Medtronic´s role in it? Having given thought to the intensifying competition from consumer-facing cardiac wearables that have flooded the market in recent years, Reitmeier says the company´s ICM line of insertable monitors still carries an advantage over the “new kids on the block.”
“Wearable sensors are complementary to the implantable cardiac monitors we have developed at Medtronic. In many cases, patients’ clinical and economic interests are better served through earlier intervention with an insertable cardiac monitor like Reveal LINQ. In other cases, it is appropriate to screen patients with other tools before moving them to a technology like Reveal LINQ. Yet, over the past 20 years Medtronic ICMs have monitored more than one million patients across cryptogenic stroke, syncope and AF management, and have shown a superior diagnostic yield leading to informed medical decisions.”