Starting a medical device company has massive risks. For Cardiac Insight CEO Brad Harlow, who loves playing poker, taking calculated risks comes quite naturally. He understands poker is just a game, but building a medical device start-up to positively impact thousands of lives is the result of years of blood, sweat and tears—and all the risks are well worth it.
Early on, Brad knew wearable ECG sensors would change the way medicine is being practiced in the market. He scrutinized existing players in the market and pondered what “pocket aces” were required to move forward in many markets.
Brad’s vision became clear. “Our aim is to completely transform ECG arrhythmia diagnosis at the point of care.” At the time, there was not a single company out there with automatic ECG analysis software or a low-cost disposable sensor. Determined, Harlow set out to build what he defined as ‘a game-changer’ in the cardiac medical device market and he met his goal.
Enter Cardea SOLO™ — the only all-inclusive ECG Arrhythmia Analysis System. Cardea SOLO consists of a wearable 7-day, single-use ECG Sensor and Windows® based automated ECG analysis software that produces draft findings right in the clinical care setting, in 5 minutes or less. The Cardea SOLO System obtained FDA clearance in March 2017.
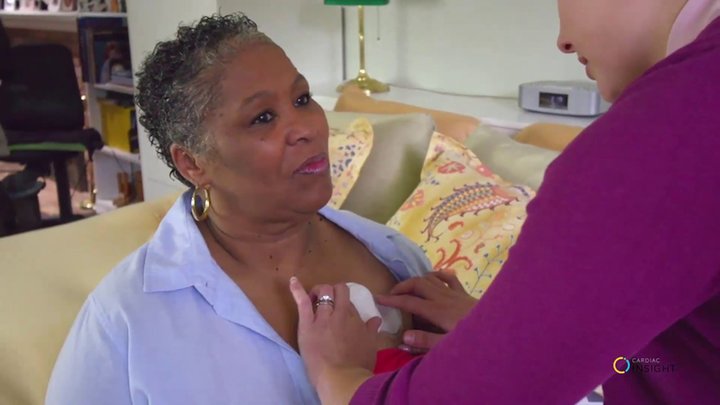
The Cardea SOLO Sensor is a wing-shaped device that houses a small CPU module, or uProcessor, and ECG chip integrated with two adhesive-backed electrodes. Once the single-channel device is applied to a patient’s chest and activated, the Sensor collects 250 samples of the heart’s electrical activity every second. If a patient experiences a bout of dizziness, light headedness, or chest pain, he or she can press an event button on the device, which will flag the incident for clinical review once the data is imported into the SOLO Software.
“It’s exciting! We are changing the way athletes and youth are screened for sudden cardiac arrest, we’re learning how extreme environments affect cardiac performance and contributing to the body of research about how individuals develop certain types of cardiac arrhythmias,”
Patients can go about their day exercising, working, and even showering while wearing the device. At the end of the prescribed wear period, the patient returns to his or her physician for a clinical interpretation of the data. Combined with their usual other assessment data, most clinicians can come to a diagnosis in 7 days or less.
Unlike traditional Holter monitors, a technology that has remained essentially unchanged in the last 60 years, data recorded by SOLO is automatically analyzed by the SOLO software right in the physician’s office; allowing clinicians to bypass expensive and timely third parties and come to a diagnosis in a matter of minutes. Moreover, doctors have unlimited access to their patient’s ECG data enabling doctors to view specific days and times to review any symptomatic events the patient recorded. Simply put, it drastically shortens the mean time to cardiac diagnosis and treatment.
Current multi-day ambulatory cardiac monitoring technologies, including other wearables, take days, if not weeks, for the same data to be analyzed, as the majority of multi-day ECG review services need to be outsourced. ECG service companies also make it more problematic and time-consuming for clinicians to access the patient’s full record of original ECG data. “Cardea SOLO customers own and manage all their own data. We don’t see it or have access in any way. This enhances patient data security and privacy, which is typically a big concern for healthcare providers and the patients they serve,” explains Harlow.
Eliminating the need for expensive and time-consuming third-party ECG service centers and putting the control of patient data, along with its analysis in the hands of physicians, has been an absolute masterstroke for Cardiac Insight. As a result, more orders are rolling in. The company sells its devices to a growing number of large physician groups, offices and hospitals across the nation, as well as cutting-edge research institutions around the world.

Members of Team USX at the Denali Summit.
Photo courtesy of Dave Ohlson, D.O
“Providing an unobtrusive, longer-term ECG wearable recorder with in-office results and reporting capabilities is really catching on as the new standard of care for clinicians who want to optimize time to patient diagnosis. Adopting Cardea SOLO can improve patient compliance and streamline what is now currently a very fragmented workflow,” emphasizes Harlow. Cardea SOLO can also be used for post-procedural observation, ongoing evaluation of therapeutic treatment and long-term care plans. Cardea SOLO can also fill a much-needed emergency room role for patients who present with symptoms suggestive of a cardiac arrhythmia.
“It’s exciting! We are changing the way athletes and youth are screened for the risk of sudden cardiac arrest, we’re learning how extreme environments affect cardiac performance and contributing to the body of research about how individuals develop certain types of cardiac arrhythmias.” commented Harlow.
Cardiac Insight’s culture of “extreme ECG innovation” to solve challenging diagnostic problems continues with its other game-changing product, the Cardea 20/20 ECG™. Cardea 20/20 ECG is the only 12-lead ECG System in the world engineered with specialized algorithms that can detect the risk of Sudden Cardiac Arrest (SCA) in athletes and youth. The National Football League, National Basketball Association, Major League Baseball, National Hockey League, and several collegiate athletic conferences including the PAC-12® and SEC®, are among the many organizations using Cardea 20/20 ECG for routine ECG screening during their pre-participation physical exams (PPE). ECG screening of middle and high school youth nationwide is also expanding, as it has shown great success in saving young lives at risk where programs are deployed.
Some intrepid mountaineers recently put Cardea SOLO to an extreme test — namely, the 20,310-foot-tall Mount Denali. In 2018, veterans and active military members along with a physician and researchers wore four consecutive Cardea SOLO Sensors during their 28-day trek to gather high-altitude cardiac performance data. “We were able to easily secure a large amount of data,” said Dr. David Ohlson, an accomplished climber and filmmaker. “The data we captured will help provide new insights into how frequently abnormal heart rhythms occur in healthy individuals at high altitude, and give us a model for larger studies in the future.”
"We are constantly learning, growing, and enhancing the capabilities of our solution to cater to the needs of the evolving medical devices market"

Brad Harlow, CEO of Cardiac Insight
Innovation on the Ground and at 20,310 ft/ 6190m

Cardea SOLO™ Wearable Sensor
The first wearable ECG sensor that provides physicians instant access to patient data, enhancing mean time-to-diagnosis and reducing the overall cost of patient care. The water-resistant, disposable sensor continuously records high-quality ECG data and integrates patient event markers throughout all daily activity.

Cardea SOLO™ Analysis
Cardea SOLO Smart Cable retrieves patient ECG data from the sensor right in the physician’s office without the need for costly and time-consuming outside data analysis centers. Recorded ECG signals are analyzed to provide comprehensive reports of heart rhythms captured for immediate review.

Cardea 20/20 ECG™
Cardea 20/20 ECG is the only system in the world designed specifically to support cost-effective cardiovascular assessment of youth for identification of risk factors associated with sudden death. The system is built upon the International Criteria for ECG Interpretation in Athletes as defined by a think tank of cardiology, electrophysiology and sports medicine experts.
A Promising Future
It is evident that Cardiac Insight—committed to innovation that addresses unmet needs and drives improved health outcomes—is ushering in a comprehensive and streamlined approach to cardiac arrhythmia diagnosis. “We are constantly learning, growing, and enhancing the capabilities of our solution to cater to the needs of the evolving medical devices market,” says Harlow. The poker afficionado will recognize this analogy, “When you have a passion to improve healthcare and hold a great “hand” like Cardea SOLO, the odds of winning will be in your favor” concluded Harlow.
Cardea SOLO for Targeted Partnerships and Clinical Trials
Spearheading innovation through partnerships with leading companies has always been atop Harlow’s agenda. Cardiac Insight partnered with VivoSense to integrate Cardea SOLO ECG with the VivoSense data platform to provide advanced insights into performance of new medicines and therapies. More subjects in private pharmaceutical and university clinical trials are also wearing the Cardea SOLO Sensor because of the improved wear-time compliance and robust data analysis tools provided by the System.
Providing an unobtrusive, longer-term ECG wearable recorder with in-office results and reporting capabilities is really catching on as the new standard of care for clinicians who want to optimize time to patient diagnosis. Adopting Cardea SOLO can improve patient compliance and streamline what is now currently a very fragmented workflow