The Pioneers
in Cardiac Arrhythmia Software and Remote Patient Monitoring
CardioComm Solutions
Etienne Grima, CEO, CardioComm
Toronto-based CardioComm was the first company to enter the personalized ECG monitoring market. With a suite of medically credible heart monitoring solutions to the consumer market, CardioComm continues to be a leader in ECG management technologies.
By Oluwaseun Oluwo
CardioComm Solutions, Inc. (TSX VENTURE: EKG) has been a pioneer in the development of proprietary and specialized electrocardiogram (“ECG”) software for the diagnosis and management of cardiac patients outside of the hospital environment under the GEMS™ (Global ECG Management Solutions) brand. The Company’s advances have been at the forefront of revolutionizing mobile and remote cardiac monitoring systems in the medical market for over twenty years and eight years in the consumer over-the-counter (OTC) health and wellness markets. Their products are sold worldwide through a combination of an external distribution and North American-based sales networks. CardioComm has earned the ISO 13485:2016 under the Medical Device Single Audit Plan (MDSAP) certification (Canada and US), is HIPAA compliant and holds clearances from the European Union (CE Mark), the USA (FDA) and Canada (Health Canada).
In 2012, CardioComm became the first company in North America to be granted FDA and Health Canada OTC clearances for developing, marketing and selling ECG devices direct to consumers without a physician prescription under the HeartCheck™ and GEMS™ Home branding. This innovative solution enables a cost effective and reliable remote monitoring interaction between individuals living their lives outside of the hospital and their doctors and health care providers.
Since CardioComm’s consumerisation of ECG monitoring and their introduction of consumer friendly ECG technologies to the market, major organizations such as Apple, Google, Verily and Withings have continued to build on what CardioComm first established. "We understand the importance of our pioneering efforts and see the value realized from advances made by those who followed our lead," states Etienne Grima, the current CEO. He also adds that "CardioComm is still a pioneer and will continue to bring innovation to the medical and OTC ECG monitoring markets."
The Company has recently championed advancements in supporting new operating systems and hardware technologies that are not expensive and are easy to use, requirements that are especially relevant with the advent of more personalized medicine being adopted by the consumer and healthcare markets with the use of mobile and wearable devices.
Cardiocomm's 4 lines of business
Patient management system (GEMS WIN) for Cardiac event monitoring and ICD/ Pacemaker management.
End users: Hospitals and physician groups
Ambulatory ECG Monitoring

Sale of thirdparty companies’ hardware that is compatible with CardioComm Solutions’ software.
End users: OEM companies
Third-party companies

Regulatory body approved, OTC ECG devices: HeartCheck Pen, Palm, Monitor, CardiBeat
ECG reading services through Smart Monitoring
End users: Consumers
Consumer-use ECG Devices

The company has supported several remote patient monitoring groups in Europe and the United States to enable their telemedicine platforms to manage ECG communication and data transfer complexities through custom cloud solutions. End users: Hospitals and physician groups
Customized Software Solutions

Detecting arrhythmias has traditionally required use of large and expensive ECG monitors only obtainable from independent diagnostic testing facilities (“IDTFs”) or medical offices and hospitals. Well, not anymore. CardioComm's HeartCheck™ devices (CardiBeat, Palm and PEN), which are small-sized, Bluetooth-enabled, handheld ECG monitors, are useful in the diagnosis of a variety of arrhythmias, such as bradycardia, pauses, ventricular premature beats and atrial fibrillation and are gaining traction for use by healthcare organizations. The pocket-sized HeartCheck™ ECG devices received regulatory clearances in 2019 and enable patients to record and define their heart health anywhere and anytime that symptoms may occur.
These Bluetooth connected HeartCheck™ devices use GEMS™ Mobile, a proprietary version of GEMS™ designed to operate as a mobile Smartphone app. GEMS™ Mobile can save recorded ECGs which are then automatically forwarded to CardioComm's SMART Monitoring ECG service with an option to forward ECGs directly into the Electronic Medical Record of the patient’s cardiologist. The devices can transmit recorded ECGs to a physician, a clinic or CardioComm’s SMART monitoring ECG reading service. The consumer or patient using the devices is also provided an ECG report through the GEMS™ Mobile app that is based on the Company’s hospital ECG software, which means that any HeartCheck™ ECG report will not only be familiar to physicians but can be used for ECG reading reimbursements by physicians.
The HeartCheck™ Solution - Extending the Reach of Physicians’ Circle of Care
The Road to Innovation
We understand the importance of our pioneering efforts and see the value realized from advances made by those who followed our lead. CardioComm is still a pioneer and will continue to bring innovation to the medical and OTC ECG monitoring markets.
CardioComm possess an extensive 20+ year history of servicing the ECG monitoring market and together with our well-trained team, we possess a unique knowledge base. There is little in the ECG world that we have not already done nor that we will not be able to do in assisting our customers moving forward.
Etienne Grima, CEO, CardioComm
The Software Play: Redefining Possibilities
For third-party device manufacturers looking to bring new medical devices to market, a GEMS™ integration can leverage CardioComm’s existing software medical device clearances to secure clearances for new medical devices faster and with lower costs. This is a key and focal corporate selling point. In 2019, CardioComm secured OTC medical device clearance for their new HeartCheck™ handheld CardiBeat ECG device manufactured by an OEM partner. CardioComm also secured Canadian Health Licensing clearance for continued access and sale of hospital/physician prescribed ECG devices compatible with CardioComm’s GEMS software from an established American device manufacturer by leveraging CardioComm’s ISO 13485:2016 certification under MDSAP where the American company did not have the MDSAP designation.
The sum benefit of these efforts is that CardioComm’s clients do not need to purchase new software platforms to have access to newly released devices nor do they need to move away from trusted legacy hardware. “We watch the market for innovation announcements, technology shifts, regulatory changes, partnership opportunities and human needs issues so that our customers stay in step with such changes and safely maintain their practice for caring for their cardiac patients," Grima comments.
In addition to providing “total” ECG solutions, CardioComm can also provide access to any component of its technologies for integration into third party platforms. This includes access to prescription and OTC ECG devices, Smart device apps, a digital ECG viewer, hospital licensed ECG management solutions, an ECG reading/reviewing/reporting service, and integration with third party medical records.
The Road Ahead: Reimagining Cardiac Monitoring
The pioneering spirit of the CardioComm team continues to drive the Company to bring new medical and OTC consumer innovations to market with the aim to improve the care of people living with or worried about cardiac disease. By leveraging their medical credibility, organizations aligning themselves with CardioComm’s products will benefit from a proven and trusted medical company versus consumer companies who are trying to make their products look like they have matched the credibility of an established medical company.
With the goal to stay ahead of technology changes, CardioComm’s ECG software development has not relented. Having identified cogent issues that still face the ECG industry, CardioComm has slated the following technologies for production this year:
- GEMS™ FLEX, an advanced ECG monitoring system that uses algorithms and AI;
- GEMS™ Triage, an automated feedback report to let OTC device users know if the ECG they recorded was of good quality and, if not, to repeat the recording to ensure a good ECG is used to rule in or rule out the presence of an arrhythmia;
- A HeartCheck™ multi-lead and Bluetooth connected ECG patch device;
- A GEMS™ based telemed solution with multiple biomarkers monitored in addition to ECGs; and,
- Compatible wearable devices such as smart garments and watches.
“CardioComm possess an extensive 20+ year history of servicing the ECG monitoring market and together with our well-trained team, we possess a unique knowledge base,” Grima points out proudly. “There is little in the ECG world that we have not already done nor that we will not be able to do in assisting our customers moving forward,” he adds.
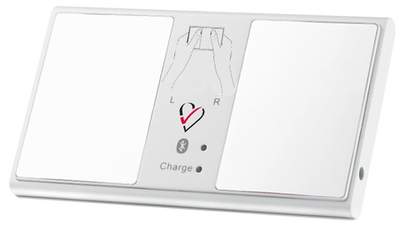
- Small handheld ECG monitor. Record medical grade 30 second single lead ECG. Available OTC.
- Connect to a smart device with the GEMS™ Mobile ECG App
- Recorded ECGs can be transmitted to a physician, clinic or the SMART monitoring ECG reading service
- Generate your own ECG record in PDF format
The HeartCheck™ CardiBeat
The HeartCheck™ Palm
- FDA Cleared. CE mark. Available OTC
- Capture Lead I or II heart rhythm recordings
- Interface with GEMS™ Mobile ECG App
- Transmit ECGs to a physician, clinic or the SMART monitoring ECG reading service or generate your own ECG record in PDF format
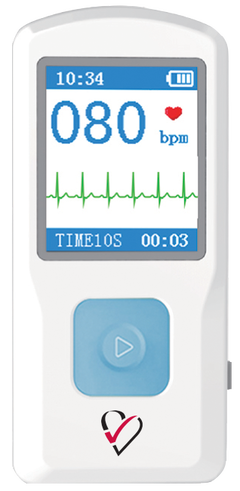
The HeartCheck™ ECG PEN
- FDA Approved for consumer use. Available OTC
- Pocket-sized PEN
- Monitor For Arrhythmias Anywhere
- Web Access to a Qualified Physician
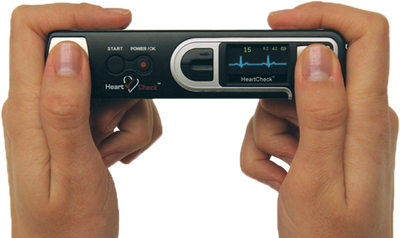
The HeartCheck™
Handheld ECG Monitor
- FDA-cleared. Available OTC
- Stores up to 200 thirty second ECG readings.
- SMART Monitoring ECG Interpretations
- Recommended for Athletes, Seniors, Nursing Home and Home Care Facilities
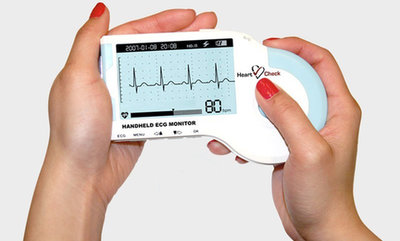

- FDA-cleared mobile app for recording and managing ECGs
- Paired with a HeartCheck™ ECG device,
- View, print and send your ECG for medical review
- Works with CardiBeat and Palm only
- Generate own PDF report
GEMS™ Mobile ECG
GEMS™ Home Flex

- FDA Cleared.
- Desktop application. Available for both Windows and Mac OS.
- Works seamlessly with the HeartCheck™ ECG devices.
- Allows to view, save, manage, and upload heart readings directly to a physician or ECG Coordinating Centre.
- Works with PEN and MD100 (Monitor)
HeartCheck™ SMART Monitoring

ECG Analysis and interpretion by a physician, ECG Coordinating Centre or both
- Report prepared by experts will be available through GEMS Home application

- Supports Third-part devices
- Efficient, scalable and easy-to-use
- Extensive and detailed standard and custom reports
- Customizable workflow
- Track and store comprehensive patient information
GEMS WIN
Software solution for Cardiac Event Monitoring, ICD/Pacemaker Follow-up & In-Clinic Pacemaker Management
GlobalCardio™ 12 Lead (GC 12)

- View ECGs (current & historical) from any where, any time, any device
- FDA-cleared editable automated interpretation algorithm
- Detailed activity logs and auto billing.
- Customizable filters, frequency, speed and calipers.
- Fully HIPAA Compliant.
A portable, web enabled ECG device that is EMR compatible.
GEMS™ Lite For Windows
- Single user, stand-alone MS Access based application
- Cost Effective ECG Receiving Solution
- Upgradeable to GEMS™ WIN Software
- Efficient and easy-to-use
- New scheduling ability

Electronic ECG Receiving System
CardioComm’s founder William (Bill) Smith spoke passionately about shifts in the healthcare space towards proactive and preventive care and the goal to develop technologies where people could be monitored. The intent was to develop technologies that could determine that if a cardiac issue was likely to happen an ambulance would pull up and tell you to get in to go to the hospital before it actually did happen. Bill’s motivation was an elderly parent who had heart disease and who lived nearby. When he heard an ambulance he worried that it has been called to his parent’s home.
Driven by this, the CardioComm team has worked to build innovative solutions to enable medical quality interactions between patients living their lives outside of the hospital and their doctors and health care providers. The company builds consumer and medical market solutions through which ECGs can be monitored remotely by a physician, clinic, or ECG coordinating centre under a workflow that cuts the cycle time down for diagnosis considerably.
For someone who has operated within the cardiology clinical research and pharmaceutical area for over three decades, Etienne Grima, the current CEO knows what matters in leadership is the ability to be proactive, dedicated, to possess excellent decision-making intuition and to lead by example.
Grima acknowledges, “If you stand still as a business when things are going well and you don't challenge yourself, inevitably your competition catches up with you. He adds, “CardioComm did lose ground with the advent of free access to Bluetooth connectivity to Smartphones and primarily the unlocking of the Bluetooth of iPhones. Soon after Smartphone connectivity became easy, several competitive companies used the CardioComm FDA clearances as predicates to their technologies.
The lost ground did not deter CardioComm and the Company has recently championed remarkable advancements to redefine their products and offerings and to remain a preferred ECG solutions provider to the evolving remote health monitoring markets. The emphasis has been placed on supporting new operating systems and hardware technologies that are not expensive and are easy to use, requirements that are especially relevant with the advent of more personalized medicine being adopted by the consumer and healthcare markets with the use of mobile and wearable devices.
Corporate leaders do rely on excellent teams and Grima believes in his. “We are a small and well-trained team with the benefit of working for a company with an extensive 20+ year history and which possesses a unique knowledge base,” Grima points out proudly. “There is little in the ECG world that we have not already done nor will be able to assist our customers to do moving forward,” he adds.
CardioComm’s total ECG solution includes over-the-counter/consumer and medical devices that connect to Smart devices, as well as software running in hospitals and a unique and valuable capability to offer rapid ECG reviewing services. It is this ability to provide a total ECG management solution comprised of critical components that may be used in whole, or in part, within other parties’ remote patient monitoring platforms that provides the company a yet unassailable edge over its closest rivals. Additionally, announcements on recent developments have shown that CardioComm is not ready to relinquish its enviable spot.
CardioComm: The Pioneer's Come Back Bid
Robust, Visionary Leadership
