iRhythm
Redefining
Arrhythmia Detection
By Gergana Koleva
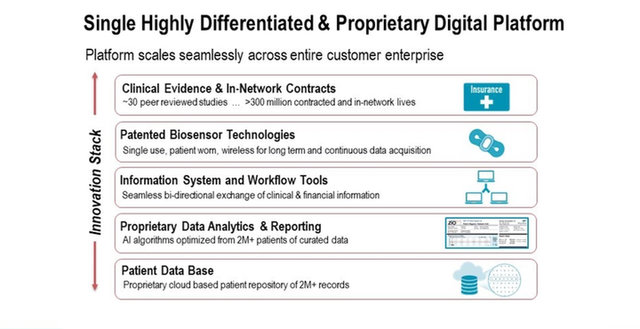
iRhythm is redefining the way cardiac arrhythmias are clinically diagnosed. The company combines wearable biosensor devices and cloud-based data analytics with powerful proprietary algorithms that distill data from millions of heartbeats into clinically actionable information.
Intelligent cardiac monitoring
As cardiac monitoring gets more sophisticated by incorporating algorithms trained on vast amounts of relevant data, companies that bring such products to market are taking care to ensure not only the overall accuracy of the models, but also their ability to detect rare or infrequent arrhythmias. iRhythm Technologies (Nasdaq: IRTC), a California-based digital health care company, has led the ambulatory cardiac monitoring with its clinically validated device in the form of a wire-free adhesive chest patch called Zio. The patch, whose biosensor detects arrhythmias in ambulatory patients, delivers uninterrupted monitoring for up to 14 days and records heartbeat data that show if, how many, how frequently, and what types of irregular heart rhythms occur, as well as their comparability to the patient´s normal heart rhythm. “With Zio, we are measuring patients ‘in real life.’ They wear it for 14 days — while eating, sleeping and showering — and if they feel a symptom, they press the [symptom-log] button on the device. We have technology that shows what type of activity was happening at the time, which gives physicians the insights on how to treat the symptoms,” says Kevin King, iRhythm´s president and CEO.
“iRhythm´s AI algorithm utilizes a massive database that contains all combinations of arrhythmias. Our database has more than 500 million hours of curated information that can be compared to a patient´s results, which teaches our AI´s neural networks how to distinguish among the various arrhythmia classes,” says King. Through a recent partnership with Verily, Alphabet´s healthcare data analytics branch, iRhythm has gained access to additional data that, together with its proprietary data pool, forms what it calls “the world´s largest heart rhythm database.” King says the partnership will allow the company to keep refining its analytics capabilities so that the algorithm´s predictions can keep improving.
“The data depository allows us to analyse information and trends and segment patient populations in ways that haven´t been done before [because] the data resides in individual practices – it hasn't been consolidated. But by having this data centralized and combined with personal health and claims information, and physician notes, we have the ability to gain new insights.”
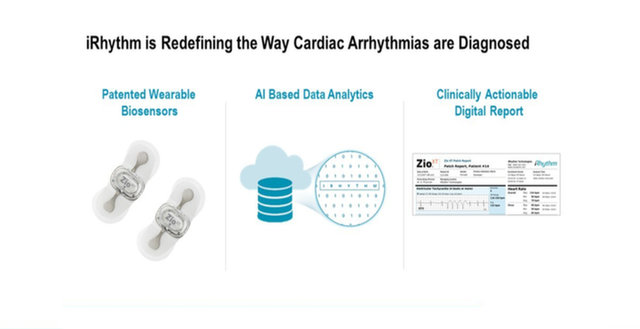
“The way that iRhythm uses AI technology helps to expedite comprehension of large ECG data sets, boosts clinical efficiency and lets physicians focus on patient care rather than having to re-look at questionable strips.”

Kevin King, President and CEO, iRhythm
The Silent AFib market
By focusing on arrhythmia detection, iRhythm aspires to improve the standard of care for cardiac patients. But more than that, it has set its sights on mastering the art and science of detecting asymptomatic atrial fibrillation (AFib).
There are 10 million patients in the U.S. who have cardiac risk factors but are yet to be diagnosed for atrial fibrillation. Asymptomatic or “silent” AFib as one may call it is associated with certain risk factors like high blood pressure, diabetes and sleep apnea – which increase an individual’s likelihood for developing the disorder. Kevin foresees iRhythm expanding into this market. “Right now we are focused on the symptomatic market, but I think the asymptomatic market will be our increased focus as the technology continues to develop. At iRhythm we´re investing in multiple areas that will help that become a reality,” says King, adding that the partnership with Verily will bring in next-generation atrial fibrillation products that combine both companies' technologies to improve the screening, diagnosis, and management of patients with asymptomatic atrial fibrillation. "This collaboration brings together our experience in AI-based algorithm diagnosis, Verily's advanced health data analytics technologies to address the millions of patients living with undiagnosed AFib," says Kevin.
The company´s commitment to developing an effective solution for early detection of asymptomatic AFib has been validated by the results of a clinical trial, published in JAMA in 2018, that showed using the Zio patch led to an AFib diagnosis in 6.7 percent of monitored asymptomatic participants compared to 2.6 percent of observed controls.
Another study, conducted in collaboration with Kaiser Permanente, that speaks to iRhythm´s advances in measuring arrhythmia showed that the Zio device correctly identified levels of AF burden at which stroke risk becomes elevated. An understudied risk factor, AF burden was defined as the percentage of time a patient wearing the Zio patch experienced AFib or flutter. It is a value that traditional non-continuous ECG sensors cannot easily capture due to their intermittent usage on patients, which prevents the collection of enough continuous data.
“Today in the U.S. and around the world, the market for testing patients with arrhythmias is mostly centered on symptomatic patients, so there is a huge market that has been disrupted by Zio. [The road ahead] is settling the storm we´ve started and making sure
we´re available to as many patients as possible,” says King, who took the company public in 2016.
We're combining our expertise in cardiology monitoring, data analytics, and commercial execution to make a tremendous impact in people's lives.
An extraordinary journey
With clinical-grade wearable ECG innovation still in its early days, iRhythm has been charting a remarkable path for itself by forging collaborations that complement its know-how in cardiac monitoring in different ways. In addition to its partnership with Verily targeting expanded health data analytic capacity and its study with Kaiser Permanente on AF burden (KP-RHYTHM Study), it has also partnered with the Stanford Machine Learning Group – the outfit led by prominent computer scientist Andrew Ng – to draw on its cutting-edge expertise in deep neural networks (DNN).
That endeavor, which used more than 91,000 30-second ECG strips from 30,000 unique Zio patch users to train and test a computerized arrhythmia classification model, produced the algorithm used in the Zio device to detect arrhythmia. The collaboration with iRhythm and the discovery it led to, published in Nature Medicine, is regarded as one of the Stanford group´s most noteworthy achievements in the field of medical AI.
For its part, in the spirit of open science iRhythm has made publicly available the independent cardiologist-labeled test dataset used to confirm the accuracy of Zio´s algorithm.
So what does the future hold for iRhythm and its peers in the cardiac ambulatory monitoring space?
“The way that iRhythm already uses AI technology helps to expedite comprehension of large ECG data sets, boosts clinical efficiency and lets physicians focus on patient care rather than having to re-look at questionable strips. I think technologies in artificial intelligence and machine learning will continue to inform how cardiac monitoring progresses,” affirms King.
Today in the U.S. and around the world, the market for testing patients with arrhythmias is mostly centered on symptomatic patients, so there is a huge [asymptomatic AFib] market that has been disrupted by Zio.

