HeartSciences
New Kid on
the Block
World´s
ECG
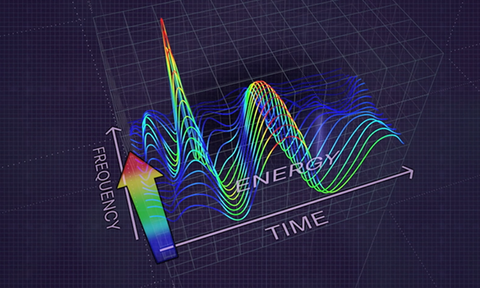
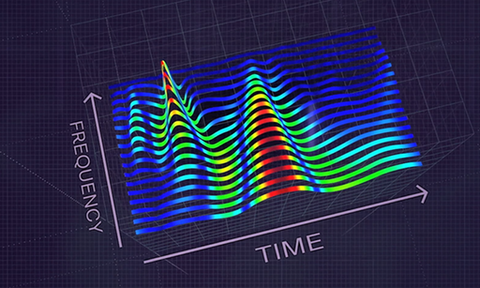
By delivering a granular heat map of the heart`s energy distribution, HeartSciences´ AI-powered tool converts a diagnostic gap in current ECG testing into an opportunity to revolutionize the way heart disease gets nipped in the bud.
By Gergana Koleva
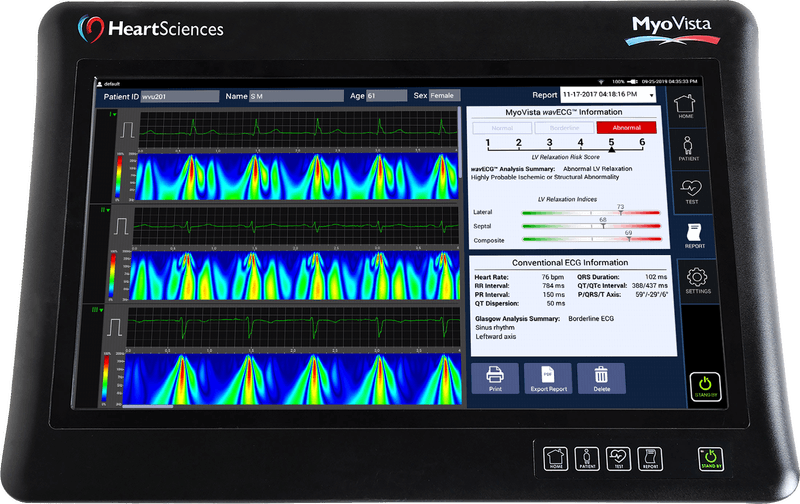
HeartSciences CEO Mark Hilz has an audacious goal and the technology to back it up: he wants the MyoVista Device to replace the conventional ECG. Displaying a graphical color scale of the heart`s spectral energy distribution, this AI-powered tool addresses a current diagnostic gap in current ECG testing capabilities and provides the opportunity to revolutionize the identification of potential heart disease.
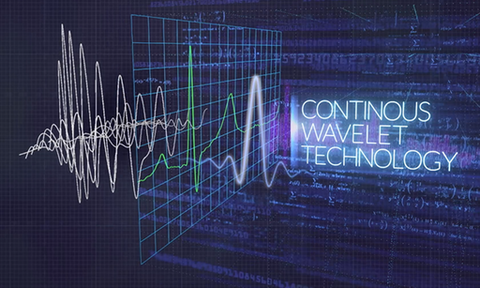
Innovation by adding completely new ECG capabilities, Texas-based medtech company HeartSciences has focused its technological expertise on detecting relaxation abnormalities related to the heart´s left ventricular (LV) diastolic function, an early sign of most forms of heart disease. Its initial product, the MyoVista Wavelet ECG (wavECG) Cardiac Testing Device, is a 12-lead ECG device whose distinctive quality is the use of AI and advanced signal processing called continuous wavelet transform to obtain frequency information. Conventional ECG devices focus only on voltage over time. MyoVistas’ sensitivity of 85% and a specificity of 74% for the detection of abnormal LV relaxation adds new valuable capabilities compared to current ECG technology. Although highly effective at detecting arrhythmias, the conventional ECG has significant limitations related to the detection of structural and ischemic heart disease.
Hilz says the MyoVista Wavelet ECGs advanced capabilities set the company apart from other electrocardiology innovators by adding new capabilities that are normally done through echocardiology based testing and significantly improve front-line ECG testing for heart disease.
“ECG technology has been around a hundred years and it is the frontline tool for detecting heart disease along with doctors assessing patient specific risk factors. Currently the majority of patients that undergo more expensive cardiology based testing have a negative test outcome, which means the healthcare system is spending significant amounts on unnecessary testing. While the vast majority of patients that have heart disease still find that out through an adverse event, such as angina or a heart attack, rather than through frontline testing. This is due to a diagnostic gap in front-line testing capabilities,” he explains, highlighting the missing link between the current screening and diagnostic methods that sparked the idea behind MyoVista.
To address that diagnostic gap, the MyoVista Device was developed on the vision that by using artificial intelligence and signal processing to process the extracted ECG information from the underlying conventional ECG trace it can obtain a far more detailed view into a patient´s heart function as compared to a conventional ECG. Hilz says MyoVista Technology more closely correlates electrical changes picked up by an ECG test with structural changes related to the early signs of heart disease.
“We started by asking ourselves what conventional ECG frontline testing is good at as well as what are things could be improved upon. The ECG is really good at detecting whether a patient has an electrical abnormality – but that´s just one of three types of heart disease. The other two types are structural and ischemic heart disease. HeartSciences was started to improve front-line testing for structural and ischemic heart disease,” says Hilz.
Frontline innovation
One of the problems we saw in the ECG industry was that the vast majority of patients who go for expensive or advanced diagnostic testing have a negative test outcome, which means they didn´t need to have that testing. On the other hand, the vast majority of patients that do have heart disease still find that out through an adverse event and not through frontline testing. That presents a massive hole in frontline testing.
Mark Hilz, CEO of HeartSciences

As HeartSciences awaits FDA clearance to market the MyoVista in the U.S., its European commercialization is already underway after having received the CE mark of approval in 2017. Although the European version is more static than the one contained in the FDA submission and does not include the latest AI enhancements, HeartSciences continues to partner with key opinion leaders around the continent who are assisting with further development of the technology.
Hilz says the most rewarding experience that has come out of the European foray has been the opportunity to interact with physicians and listen to what they want in an ECG device, and how can we change current ECG technology to be more useful to them. He credits those early learnings with showing just how big the need really is for a better front-line tool.
“While in the U.S. we have a cost problem, in Europe wait times can be a problem. So care teams there are saying, `How can I triage the waitlist to see who should go to the front of the list and go on to further testing, and who does not need to go immediately?´ In Europe there is a significant need for improved front-line testing to help them triage that list, he says.
Early learnings
The premise of why HeartSciences was started was to improve frontline testing for structural heart disease.
Back in the U.S., where healthcare cost concerns are increasingly creating an imperative to more effectively manage costs. Health care providers are looking to identify heart disease earlier while reducing unnecessary testing. HeartSciences MyoVista device provides all standard 12-lead ECG testing and therefore is compatible with existing insurance billing codes. The MyoVista device provides both wavECG analysis and conventional ECG analyses as independent test results in a single patient test. This two-in-one approach is significant because the device´s novel wavECG Technology, aimed at improving the detection of structural and ischemic heart disease, allows for the same lead placement protocol and does not require retraining of the personnel that are administering the test.
Economic value
MyoVista´s two-in-one approach is significant because the device´s novel wavECG technology, aimed at detecting structural or ischemic heart disease in asymptomatic patients, would likely not have been covered by health insurers if it were not “wrapped” into traditional ECG tracing, since it is considered an advanced diagnostics tool – yet most payors discourage the use of such methods in asymptomatic patients.

For Hilz, being able to improve a key frontline tool that can save lives is HeartSciences´ main goal. And, buoyed by the company´s initial interest in Europe and his team´s diligent efforts to incorporate physician feedback into the design of the AI-boosted version, he makes this prediction: “Along with the 12-lead functionality of a conventional ECG, MyoVista will provide physicians will additional information as a result of signal processing and artificial intelligence. This technology will be a standard feature on all ECGs in the next 10 years.”
Road ahead