Diagnostic assessments that help physicians decide on proper treatment for stenosis keep incrementally evolving, but this is not good enough for CathWorks CEO Jim Corbett and his team. Since 2018, when the Israeli company – with headquarters in both Israel and California – received FDA 510(k) clearance for its signature invention, the FFRangio System, it has been on a trajectory to disrupt the scene by introducing highly accurate, cost-effective 3D percutaneous coronary intervention (PCI) guidance that does away with the inconvenience of conventional FFR pressure wires.
CathWorks
From Visual Assessment to
Data-Driven Insight
As 2D black-and-white angiography gives way to fractional flow reserve for guiding percutaneous coronary intervention procedures, CathWorks´s FFRangio System is pushing boundaries still further by combining artificial intelligence and computational science to deliver comprehensive and powerful decision-tools. Its non-invasive technology aspires to nothing less than to transform the way physicians diagnose and assess coronary artery disease.
By Gergana Koleva
Jim Corbett, CathWorks CEO
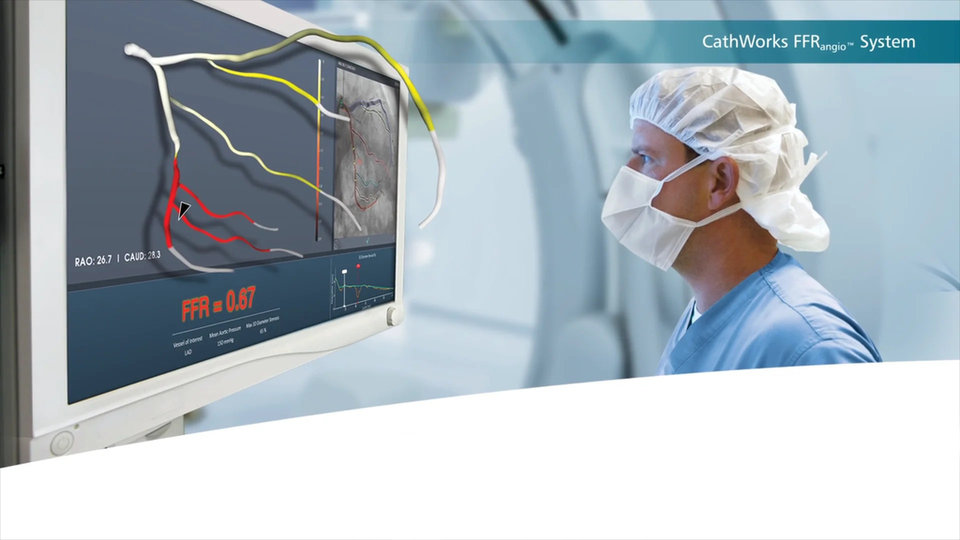
Interventional cardiologists know that not every angiogram-based visual assessment of coronary artery disease warrants a stent and that not every angiographic appearance of mild stenosis means an intervention isn´t necessary, either. Conventional FFR, which objectively measures the functional or physiological impact of the stenosis, largely solves this ambiguity, but raises other problems: elevated clinical risks, increased radiation exposure, and lengthy procedure times due to the use of invasive pressure wires. FFRangio overcomes the limitations by leveraging a combined approach of using routine 2D angiogram images, artificial intelligence, and advanced computational science to aid cardiologists in determining whether an observed stenosis is physiologically significant, all without pesky pressure wires.
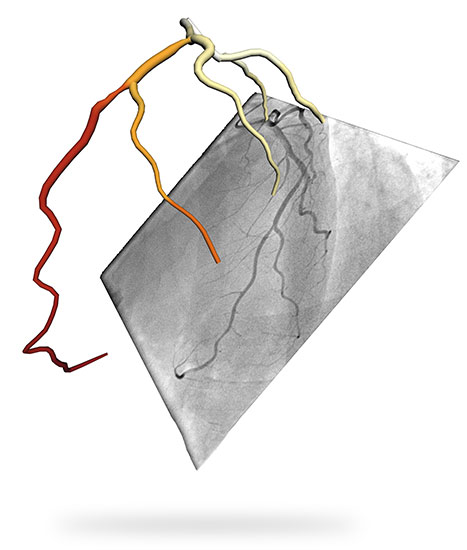
To do so, the technology uses the angiography system in the cath lab where it is being deployed, creates a 3D reconstruction of the coronary tree, and recognizes vessel geometries and patterns thanks to its proprietary AI algorithm. The coronary tree is then modelled like an electrical circuit, in which individual vessels act as resistors, and the algorithm “gets to work” again to solve millions of equations and determine flow and flow blockages at every point along the vessels in the coronary tree. The result is an interactive 3D model with comprehensive multi-vessel anatomical and physiological information.
The system effectively replaces the need for wire-based FFR, which despite being diagnostically superior to coronary angiograms remains underutilized due to the invasiveness of wire placement, secondary risks to patients, longer procedural time, and higher associated costs. Nevertheless, Corbett says CathWorks´ platform could be much bigger than the current standard-of-care FFR method.
“FFRangio provides considerably more diagnostic insight than is provided by invasive pressure wires. And because it’s non-invasive, we feel the technology could expand the use of objective physiological guidance, which will have a huge impact physicians and patients worldwide. ”
The FFRangio System is compatible with all major C-Arm machines used in angiography and easily integrates within the cath lab workflow. The analysis takes approximately 5 minutes from first click to output, that is, from processing the angiogram images to delivering an assessment – a significant time gain compared to traditional wire FFR, which can take up to 15 minutes.
FFRangio is different
The testimonial about FFRangio´s capabilities offered at TCT by a Stanford University Medical Center physician, whose cath lab has adopted its usage, reflects on CathWorks´ broader market strategy. Corbett says its approach is simply based on providing practitioners with a better decision tool that responds to the latest clinical advances and evolving patient expectations for personalized and optimized care.
“FFRangio carries significant benefit to physicians, patients, and the healthcare system as a whole. Our go-to-market strategy is focused on developing partnerships with clinicians who are champions for the advancement of patient care. ,” he explains.
Following FDA approval and series C financing round, the company made a strategic decision to focus its initial go-to-market strategy in California. Since then, they’ve achieved some significant milestones, including Japan PMDA approval and FDA approval for a significant update to the FFRangio user interface. The company is currently in the process of expanding its commercial operations globally.
In line with its international growth ambitions, FFRangio obtained FDA approval largely thanks to the findings in a multi-center international clinical trial, called the FAST-FFR trial and published in the peer-reviewed journal Circulation in the fall of 2018 – about three months prior to the FDA clearance. The study, which enrolled over 300 patients with single- or multi-vessel coronary artery disease at 10 clinical sites in the U.S., Europe, and Israel, compared the diagnostic performance of CathWorks´s product against traditional wire-based FFR in routine clinical practice. Its conclusion was unequivocal: with 91% sensitivity, 94% specificity, and 92% diagnostic accuracy, “FFRangio has the potential to eventually replace wire-based FFR measurement and substantially increase physiological coronary lesion assessment in the catheterization laboratory, thereby leading to improved patient outcomes.”
Go-to-market strategy
FFRangio provides considerably more diagnostic insight than is provided by invasive pressure wires. And because it’s non-invasive, we feel the technology could expand the use of objective physiological guidance, which will have a huge impact physicians and patients worldwide.
Jim Corbett, CEO, CathWorks
Corbett does not worry much about the competition because, as he sees it, the image-based FFRangio System is ahead of the curve and is uniquely positioned to disrupt existing diagnostic solutions, significantly improving the way coronary artery disease is treated.
“Our largest opportunity is encouraging more physicians to use physiological guidance instead of visual assessment of angiograms alone. As found in the FAME trial, visual angiography is a poor predictor whether the stenosis is blocking blood flow –the disagreement between visual stenosis and physiological significance can be as high as 50%,” he says, citing another key clinical trial published more than a decade ago in the New England Journal of Medicine that demonstrated the superiority of wire FFR to 2D angiograms. “Guidelines suggest routine use of FFR to guide decisions and now FFRangio makes attaining that data non-invasive and practical for every patient.”
Competition

FFRangio has the potential to eventually replace wire-based FFR measurement and substantially increase physiological coronary lesion assessment in the catheterization laboratory, thereby leading to improved patient outcomes.
FAST-FFR Trial Conclusion
