NuVera
New Visualization Era for
Cardiac
Interventions
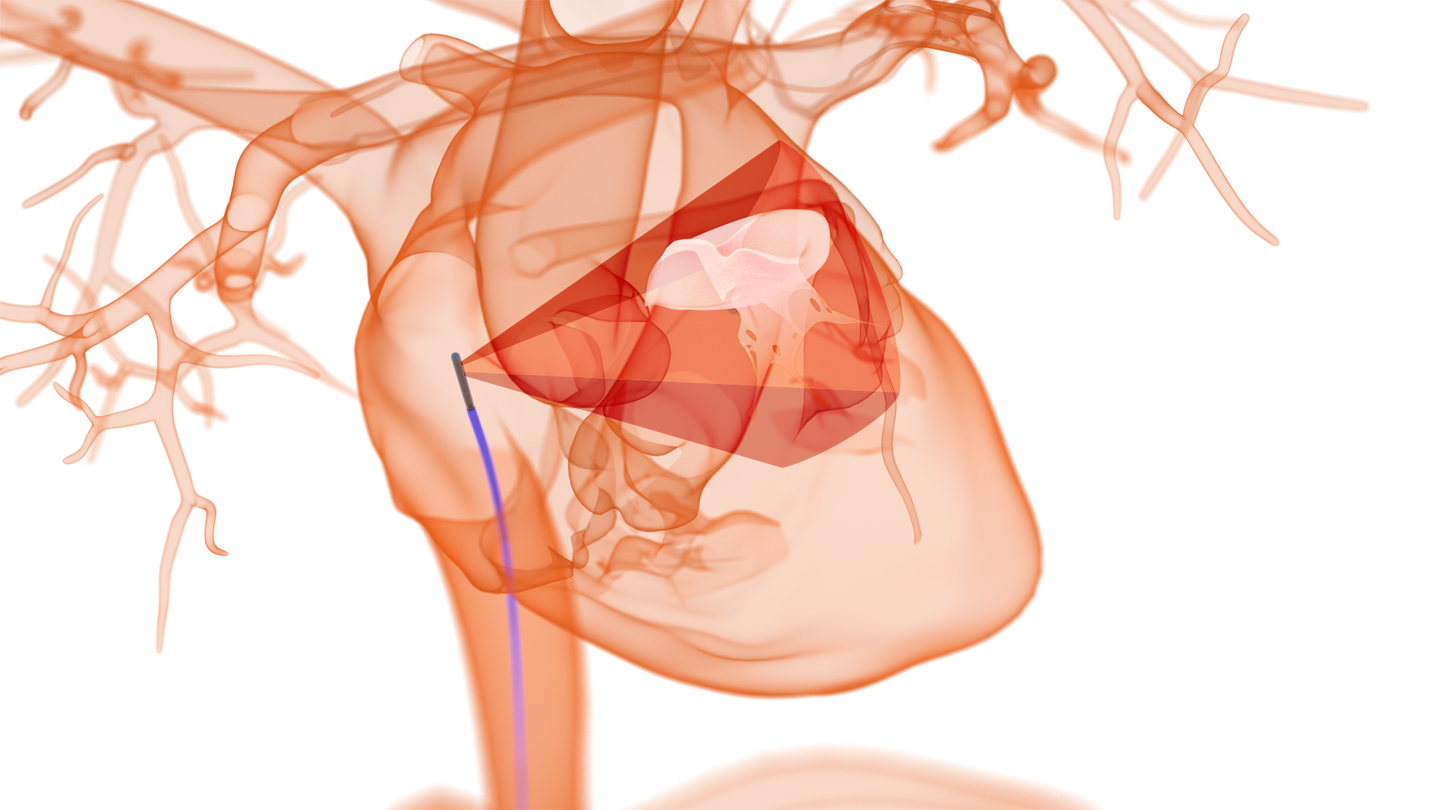
Gone are the days of fluoroscopy and 2D intracardiac echocardiography – for NuVera, at least. This California start-up is reinventing the imaging modality for complex cardiac transcatheter procedures by improving upon both the conventional tool and other 4D ICE catheters.
By Gergana Koleva
Therapeutic advances in interventional cardiovascular medicine – electrophysiology and structural heart – often steal the spotlight at medical congresses and in high-profile medical journals, and almost always so in the popular press. But these advances do not happen in a vacuum, as they depend on similarly advanced visualization tools for procedural guidance. Unfortunately, diagnostic imaging equipment and modalities have not kept pace with the speed of progress and the vast majority of electrophysiologists performing ablation – up to 77% of them – still rely on 2D intracardiac imaging, while structural heart operators lean on 4D transoesophageal echocardiography (TEE), which comes with its own challenges.
In this context of two-speed innovation, NuVera – a portfolio company of the Silicon Valley medical technology innovation hub Shifamed – sees a necessity and an opportunity: to upgrade current visualization technology so as to allow physicians to better assess complex cardiac structures, with the potential to improve outcomes and reduce procedure times and fluoroscopy exposure. Its novel tool-in-development, the NuVera™ ICE Catheter, aims to fulfill this tall order.
“Moving from 2D ICE to 4D ICE is like moving from standard printing to 3D printing,” says the start-up´s CEO Todor Jeliaskov, a strategic leader and medical technology innovator with over 20 years of experience solving problems at complex enterprises and start-up organizations. “2D ICE is widely used despite being a dated technology with limited single-plane imaging. One of the emerging areas in ICE is the introduction of real-time 3D imaging, which is referred to as 4D ICE. We believe 4D ICE is the next advancement for cardiac imaging, and we are thrilled to be a leading player in this space.”
Beyond streamlining interventions and improving outcomes, 4D ICE technology can also play a critical part in the adoption of increasingly complex structural heart procedures, such as left atrial appendage (LAA) closure, mitral valve repair, and tricuspid valve repair. To perform these procedures, physicians currently rely on 4D TEE, which requires a dedicated echocardiographer to navigate the probe and generate the cardiac images. NuVera´s 4D imaging on an ICE platform is designed to obviate this need by putting control of the image generation in the hands of the interventionalist, allowing him or her to guide the imaging catheter throughout the procedure.
Procedural companion
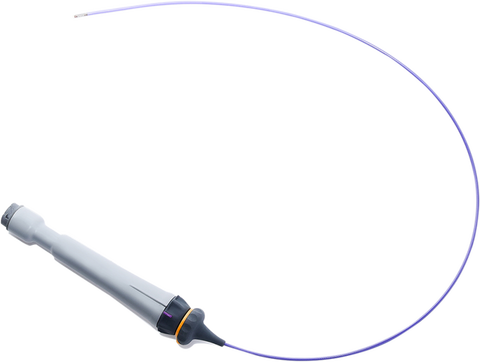
While NuVera´s catheter is still in the research and development stage and not available for sale in any country, the technology is highly differentiated from existing catheters on the market and expects to receive FDA clearance this year.
Its edge against competitors lies in its compact and powerful specifications: a 10 Fr steerable catheter with tissue penetration capacity greater than 15mm, a wide 90° x 90° field of view and state-of-the-art 2D and 4D spatial resolution, which is designed to allow for superior imaging compared to conventional 2D ICE catheters. Judged on dimensions alone, it also surpasses the current market leader in 4D ICE imaging, Siemens’ ACUSON AcuNav Volume ICE Catheter, which comes in at 12.5 Fr and a smaller field of view of 50° x 90°.
In addition, NuVera´s catheter is designed to give the operator more control with its dual-shaft design that allows for independent distal tip rotation and tip extension.
Last but not least, the NuVera ICE Catheter is also designed to provide the imaging capabilities of 4D TEE via intracardiac echo, allowing procedures to be guided by conscious sedation rather than by general anesthesia. This is a crucial part of its competitive strategy, rolling together the impacts on anesthesia-associated hospital costs, time, image quality, and patient experience under a common denominator.
“For structural heart procedures, most operators rely on 4D TEE for real-time 3D visualization, which requires a dedicated sonographer and is typically done under general anesthesia, adding cost and time to the procedure along with an increased length of stay. Providing 4D imaging on an ICE platform offers the potential to reduce the hospital’s cost burden and puts control of the imaging catheter into the hands of the interventionalist. For many procedures, such as those in the right side of the heart (e.g., tricuspid valve repair), 4D ICE is expected to dramatically improve image quality compared to 4D TEE,” says Jeliaskov.
Jeliaskov´s observations are pertinent given the ongoing gradual shift from using general anesthesia to conscious sedation for some structural heart procedures, such as TAVR, due to the shorter procedure time, earlier ambulation with better and shorter recovery time, and reduced length of stay associated with the latter. “We expect our 4D ICE catheter will further enable the shift to conscious sedation because it provides similar imaging capabilities of 4D TEE on an ICE platform,” he says.
Product innovation
At NuVera, we have a rigorous process of new product development. We work closely with clinical advisors and physician leaders very early on and continually throughout development for feedback. Recently we invited a group of physicians to our new facility. It was great to have them on-site, touching the product and sharing their thoughts on the best usage.
Involvement of physician leaders for research collaboration or R&D advice is a habitual practice in the pharmaceutical and biotech sector, but perhaps less ostensibly so in the medtech innovation space. Yet the team behind NuVera´s revolutionary new catheter is fully aware of the importance of user involvement in the research and design of its technology and the company has sought to engage physicians from day one. As a result, the technology has been designed and developed with the full participation of cardiac interventionalists and electrophysiologists, making sure that the final product will respond to operator needs and preferences that current imaging devices don´t address.
“At NuVera, we have a rigorous process of new product development. We work closely with clinical advisors and physician leaders very early on and continually throughout development for feedback. Recently we invited a group of physicians to our new facility. It was great to have them on-site, touching the product and sharing their thoughts on the best usage. We look forward to doing more of these physician meetings in the future,” says Jeliaskov.
As a case in point, one of the features of conventional 2D ICE imaging that NuVera understood physicians would be happy to do without is fluoroscopy.
“It is our goal to fully support physicians as they move away from fluoroscopy and can operate in a fluoro-less lab. We believe that physicians are looking for technologies that can provide them with less radiation exposure and less contrast for their patients. NuVera is thrilled to be a part of this change as we are creating the next generation cath lab,” Jeliaskov affirms.
Physician involvement

Aside from having invested heavily in physician market research, NuVera has also been setting the foundation for real-world piloting of the 4D ICE catheter once it receives the anticipated FDA clearance. The company has developed a U.S. commercial plan and a targeted list of accounts for the initial launch, which includes more than 65 high-volume sites for both electrophysiology and structural heart interventions.
Last September, Drs. Jason Rogers and Azeem Latib presented on the company’s flagship product at Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific road show of the Cardiovascular Research Foundation, which is the leading educational meeting in the field. In addition, the company plans to initiate two clinical studies in the first half of 2020 to further evaluate the technology and build a body of evidence.
Jeliaskov is convinced that the piloting of the device this year will mark a clear “before and after” in the evolution of cardiac imaging.
“We believe the NuVera ICE Catheter will positively impact the safety and efficiency of electrophysiology and structural heart procedures. The name NuVera was inspired by the combination of the words new, view, and era because our technology enables a new era in cardiac imaging.”
Laying the ground for post-FDA clearance


Moving from 2D ICE to 4D ICE is like moving from standard printing to 3D printing. 2D ICE is widely used despite being a dated technology with limited single-plane imaging. We believe that 4D ICE is the next advancement for cardiac imaging, and we are thrilled to be a leading player in this space.
Todor Jeliaskov, NuVera President and CEO

