FEops
Heart Interventions
Predictive Planning of Structural
FEops is reinventing structural heart procedures by improving how implantable devices are designed and tested and by guiding physicians on their optimal sizing and placement in the patient´s body. Headquartered in Ghent, Belgium, this startup sits at the junction where device-makers, medical imaging companies, and physicians unite around one goal: delivering optimal health outcomes for patients.
By Gergana Koleva
FEops is in the business of running patient-specific simulations for minimally invasive structural heart interventions. By deploying cutting-edge computational simulations to predict heart-device interactions, it aims to become a game-changer in how transcatheter structural heart interventions are planned in the future.
“The challenge for physicians with minimally invasive procedures is that they don´t see the heart anymore, so they have to rely on medical imaging to decide which implant type and size to put for the particular patient. What we have developed is a revolutionary planning technology that can take these images, make a computer model of the patient, and preoperatively test different scenarios. Based on that, the physician can make a decision on what is the optimal implant size and position for that particular patient,” says Matthieu De Beule, FEops´ CEO and co-founder, who took the company from an academic spin-off to a venture capital-backed star in the medtech galaxy.
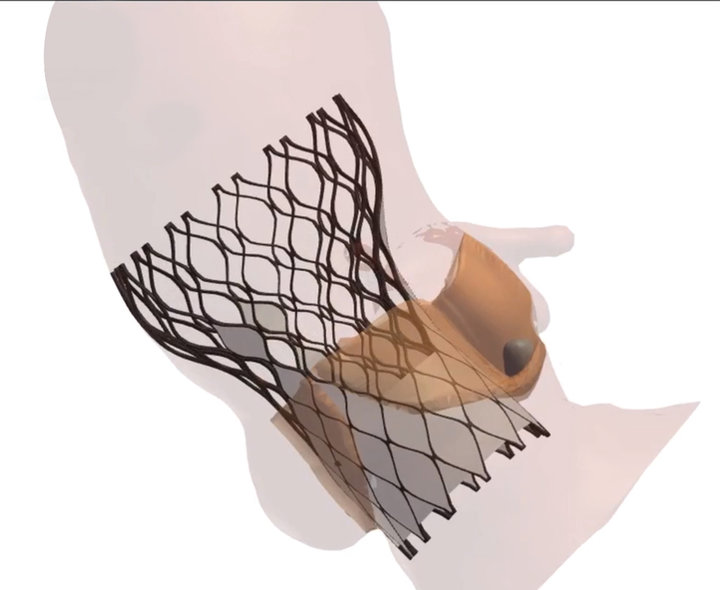
FEops´ signature product, FEops HEARTguide, is a one-of-a-kind cloud-based procedure planning environment for structural heart interventions that provides physicians with unique insights into the interaction between transcatheter structural heart devices and specific patient autonomy, using novel computational modelling and simulation technology. The current release includes workflows for transcatheter aortic valve implantation (TAVI) and left atrial appendage occlusion (LAAO) procedures, which in recent years have increasingly replaced open-heart surgery.
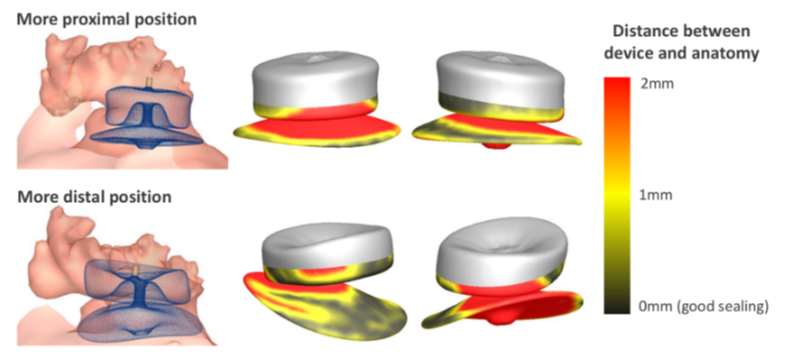
The technology works by pre-operatively juxtaposing individual patient heart measurements against a series of scenarios in terms of device sizing and positioning in order to find the optimum configuration and thus minimize risk of potential complications. It achieves this by performing finite element-based calculations, which incorporate the material properties of the device and the material properties of the patient-specific heart anatomy in order to predict how the two will interact with each other. The company´s name is itself an allusion to the methodology: FEops stands for “finite element optimal solution” – an approach to solving engineering and mathematical problems related to structural analysis, heat transfer, fluid flow, and electromagnetic potential. Nico Uwents, FEops´ director of business development, underscores the value of automating these processes in the context of minimally invasive interventional cardiology, where physicians trade a direct view of the heart for a more sparing but partially blindsided approach.
“For cath lab clinicians, what this means is that they know exactly where to go and which size to take. It means potentially less re-sheathing and mis-sizing during procedures and contributing to better patient outcomes. And thanks to standardizing the pre-op planning stage, FEops HEARTguide also helps to accelerates the learning curve for new implanters so that they can adopt it more easily and potentially start operating on more complex cases,” says Uwents.
Having obtained the European Union´s CE mark and Canada Health approval for pre-operative planning of TAVI and LAAO procedures, FEops HEARTguide is commercially available in Europe and Canada, and awaiting FDA clearance within the coming year.
FEops HEARTguide´s predictive pre-op planning capabilities enable an automated, faster, and more accurate way of completing minimally invasive procedures. Its centralized analytic functions streamline workflows and remove operator subjectivity in resolving some of the most ambiguous situations that may arise in the cardiac cath lab.
Two concrete situations in which FEops´ patient-specific computer simulations have demonstrated its utility in resolving doubts involve high-complexity TAVI and LAAO interventions. In TAVI, a procedure originally intended for elderly and high-risk patients, but which is increasingly being extended to younger, lower-risk patients with more challenging anatomies, FEops HEARTguide helps operators plan procedures for patients with bicuspid aortic valve stenosis, a condition whose incidence is higher in younger populations. In LAAO, FEops HEARTguide helps resolve the challenge of determining the exact anticipated “landing zone” of the closure device and, working backwards from there, selecting the most appropriate device size.
“If you ask physicians for LAA, they´ll say ´The procedure itself is not always the biggest concern, but you never know when you start what you're going to get. You cannot see it before you start.´ With our technology, you know exactly before you start what to do, where to go, from which size to select and where to position,” says Uwents.
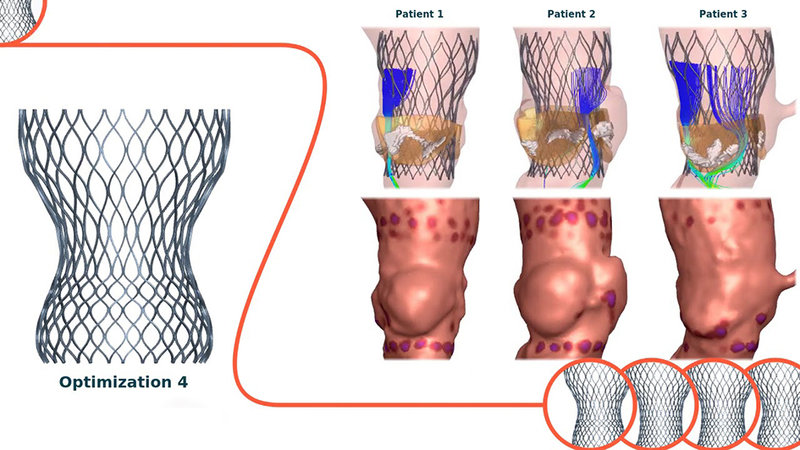
Parallel to being of service to physicians, FEops is actively collaborating with medical device manufacturers help improve the design of their products and even help them decide which prototypes to develop. In fact, The company is curating a diverse database of real patients´ heart anatomies – the patients whose cases FEops HEARTguide has “worked on” side by side with cardiologists in the cath lab – and is leveraging high customized insights derived from these data to test in silico the possible and likely interactions between device prototypes and different heart anatomies.
“Usually device manufacturers will develop two or three prototypes, then do bench testing, then animal testing, which can take some time. With our system we can help them build virtual prototypes – not one or two, but multiple – and virtually implant these prototypes in virtual replications of real patients´ anatomies to see how they interact. The simulations will check for sealing, conduction abnormalities, and leakage, among other aspects, to define which prototype is most likely to produce the best clinical outcome. This accelerates the whole product life cycle, starting with the development stage,” explains the process DeBeule.
A similar logic and process apply to FEops´ involvement with clinical trials for implantable cardiac devices. Whereas trial sponsors tend to apply very strict patient inclusion criteria – in part because they don´t want to take risk with participants in already fragile health, and in part because they enter novel un-explored territories – FEops says its simulations can predict what the outcome for implanting potentially excluded patients with the experimental device would be. In effect, the company can leverage its modelling techniques to helping medical device manufacturers to select the right patient for the right device size and position (and the other way around)
Drawing on its teams´ combined expertise in biomedical engineering, computational modelling, and simulation techniques, FEops´ unique vision has opened a world of possibilities for improving the design of transcatheter devices, the representativeness of clinical trials participants, and the ability of cath lab operators to handle the most challenging procedures. The common denominator underlying these advances is predicting how patients´ individual anatomies will react to implants – but more than that, it is a novel concept of what personalized predictive medicine in interventional cardiology will look like.
FEops HEARTguide
Clinical use cases
Design-thinking with medical device manufacturers

FEops’ mission is to create unique, predictive, personalized computational modeling and simulation solutions for structural heart interventions that empower medical device manufacturers to bring superior products faster to the market and enable physicians to improve clinical outcomes. FEops’ vision is to become the game-changer in the way transcatheter structural heart devices are designed and procedures are planned in the future.
Matthieu De Beule, CEO, FEops
